Bag-in/Bag-Out Filter System | Zero-Leak Containment | ISO 14644 Pharma & Biosecurity
Product Details:
| Place of Origin: | Guangdong, China |
| Brand Name: | MRJH |
| Certification: | CE,OSHA,ISO,ULPA,FDA GMP |
| Model Number: | Bag-in-bag-out filter |
Payment & Shipping Terms:
| Minimum Order Quantity: | 1 |
|---|---|
| Price: | Contact Us |
| Delivery Time: | 7-15 working days |
|
Detail Information |
|||
| Custom Made: | Support | Official Website: | Www.ffu-cleanroom.com |
|---|---|---|---|
Product Description
Bag-in/Bag-Out Filter System | Zero-Leak Containment | ISO 14644 Pharma & Biosecurity
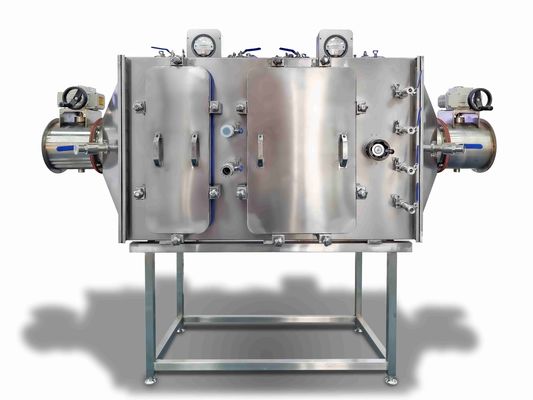
The Bag-in/Bag-Out (BIBO) filter device is a specialized containment system designed for safely handling hazardous substances, such as toxic dusts, radioactive aerosols, or high-purity particles, where minimal operator exposure and zero leakage are critical. It enables the safe replacement of contaminated filters without direct contact between operators and hazardous materials. The system features a sealed, negative-pressure enclosure with replaceable inner and outer bags: when filter replacement is needed, the used filter is sealed within an internal bag, which is then enclosed by an external bag, ensuring contaminants remain trapped throughout the process. This design is pivotal in industries where exposure to harmful agents could pose severe health, environmental, or regulatory risks.
| Product Name | Bag-in-bag-out filter |
| Air volume | 3400CMH |
| Box material | All 304 stainless steel |
| Applicable temperature | Max. 60°C |
| Purification efficiency | 99.99% |
| pressure | ≥2500Pa |
| Plate thickness | 2.0mm |
| Composition structure | Safety sealing valve + disinfection interface + primary filter + sealing door + high efficiency filter + leak detection mechanism + differential pressure gauge |
| Applicable environment | It is widely used in personnel protection and environmental protection in pharmaceutical, environmental protection, chemical, medical and health, quarantine and epidemic prevention departments. |
Key Features & Advantages
1. Absolute Containment
2. Operator Safety
3. High Efficiency Filtration
4. Flexibility & Adaptability
5. Durability & Low Maintenance
Application Scenarios
1. Nuclear Industry
2. Pharmaceutical & Biotechnology
3. Chemical Manufacturing
4. Semiconductor & Electronics
5. Research Laboratories & Hazmat Handling
6. Aerospace & Defense
![]()
![]()
![]()
FAQ 1:
Q: Does this system prevent operator exposure during viral filter changes in BSL-3 labs?
A: Yes, triple-seal gaskets and negative pressure interlocks achieve 0.001% leakage (tested per NSF/ANSI 49).
FAQ 2:
Q: Can it handle 24"x24"x12" HEPA filters with rigid frames?
A: Adjustable clamp rails accommodate 20"-36" filter sizes, including rigid/pleated designs (max 50kg).
FAQ 3:
Q: How to decontaminate after handling Category 3/4 biohazards?
A: Integrated VHP ports enable in-situ sterilization cycles (validated for 6-log reduction).